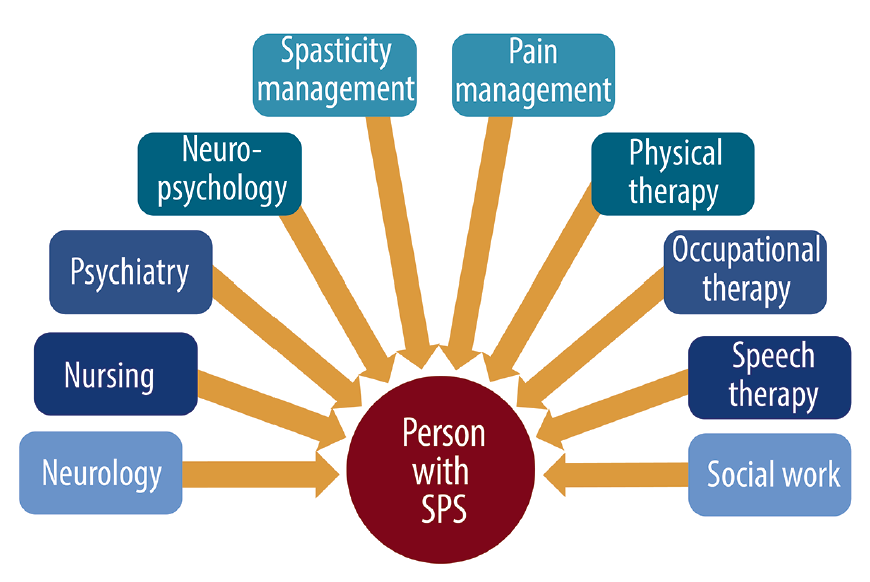
More research on the use of IVIg for symptoms of this disorder is needed. Shazie 8 years ago 38.
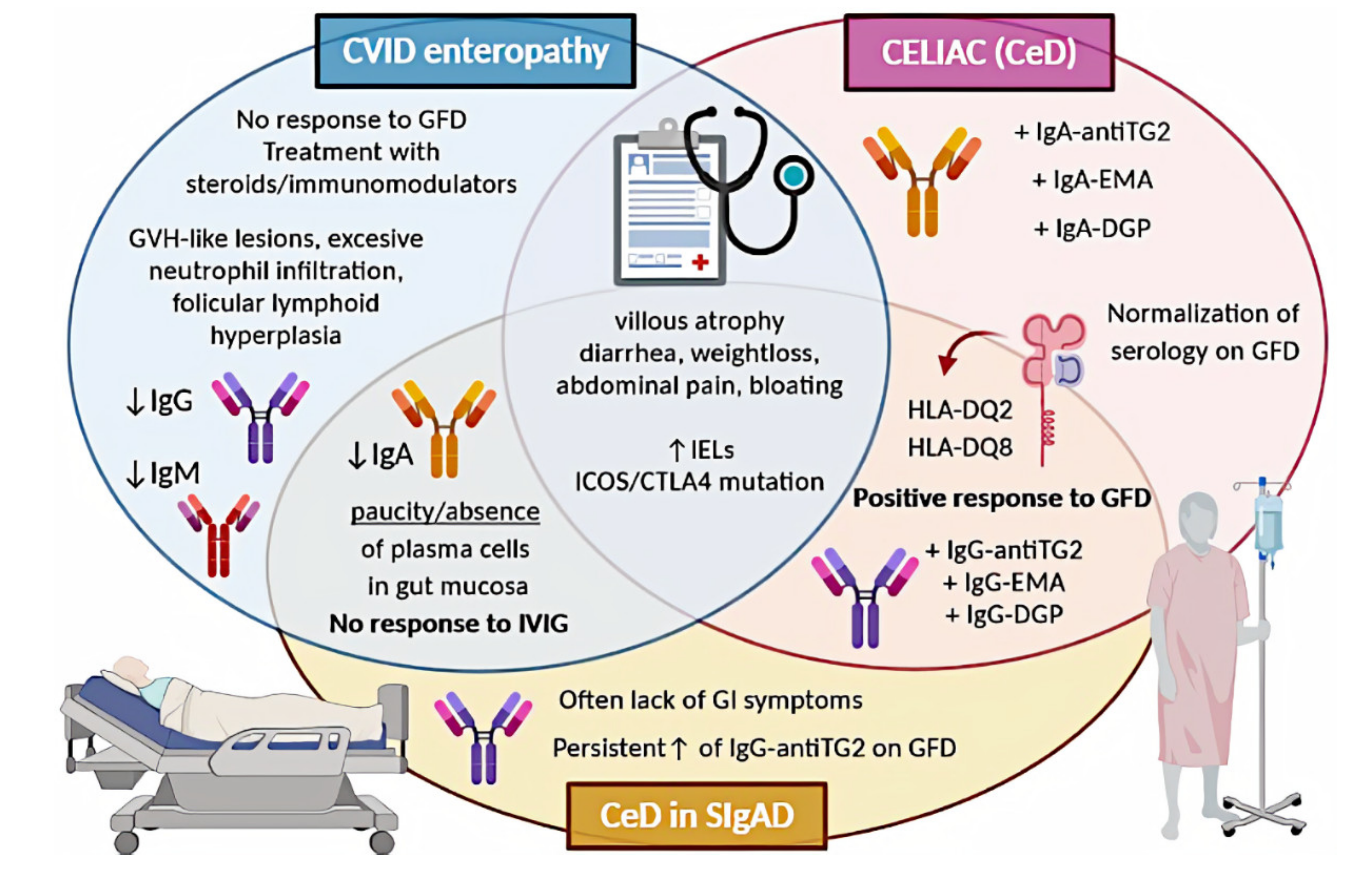
Gastrointestinal Diseases In Primary Immunodeficiencies Encyclopedia Mdpi
Arthritis is an inflammation of the joints that.

. Chronic inflammatory demyelinating polyneuropathy cidp a rare immunemediated disease of the peripheral nervous system has a gradually worsening or. IVIG is FDA approved for six conditions. The first two conditions listed are relevant to long haulers though most long haulers do not seem to have the symptoms of either condition such.
Arthritis Some forms of arthritis commonly affect children. Has voluntarily withdrawn from. TSO1334 100 Page 1 of 2 Intravenous Immune Globulin IVIG Reaction Chart ALL patients should receive information on potential reactions and how to report a suspected transfusion.
Other side effects include chills fever flushing flu-like muscle pains or joint pains feeling tired having nausea vomiting and rash. It is characterized by a sudden onset of any of the following symptoms. IVIG Treatment Side Effects Although most recipients of IVIG handle the treatment quite well some people do experience side effects.
Patients received either IVIg withdrawal placebo as investigational treatment or continuation of IVIg treatment control. Infusion reactions including severe allergic reactions have been reported especially in patients with IgA. IVIg also has less reported withdrawal symptoms than the treatment of CIDP with corticosteroids although it may have higher relapse rates than corticosteroids.
The symptoms of this include. What are the withdrawals symptoms of Ibrutinib. Complications I been off Ibru for a week and I been getting high fevers rashes headachesnone.
The symptoms after each infusion. For the most part these reactions are mild. 2 put a heating pad over my arm before the infusion to open the veins up and warm them.
Intravenous immunoglobulin commonly referred to as IVIg is used to treat many immune deficiency disorders and inflammatory conditions. About This Withdrawal Due to increased reports of hypersensitivity reactions such as rashes or urticaria andor wheezing Octapharma USA Inc. The increased use of intravenous immunoglobulins IVIg in the treatment of neurological autoimmune diseases has led to more awareness of adverse reactions.
IVIG independence remission was defined as absence of deterioration for at least two years following discontinuation of IVIG with patients evaluated at six- to 12-month. Though some patients find immediate long-lasting relief from their first immune globulin treatment the IVIg infusion process can have some side effects. The primary outcome was the mean change in logit scores from.
Drug reaction with eosinophilia and systemic symptoms syndrome involves delayedtype hypersensitivity reactions mediated by the adaptive immune system with organ. Then if my veins get irritated during the infusion Ill put the heating pad just above. Intravenous immunoglobulin IVIg infusion.
When he wakes up after the infusion he is experiencing throat tightening difficulty with swallowing esophageal burning thick post nasal drip and dizziness. There are reports of successful treatment of severely ill COVID-19 patients with high-dose IVIG 8586. A chill or a fever headache stomach pain feeling sick or vomiting joint pain low back pain tiredness.
Generally IVIG infusion can cause migrainelike headaches nausea and dizziness. The median time to onset of withdrawal symptoms is 2 days. Difficulty breathing chest tightness bronchospasms wheezing changes in the gastrointestinal system.
Ivig worked for a while but over time has given me increased raynouds attacks of worse severity and further narrowing of my blood vessels.
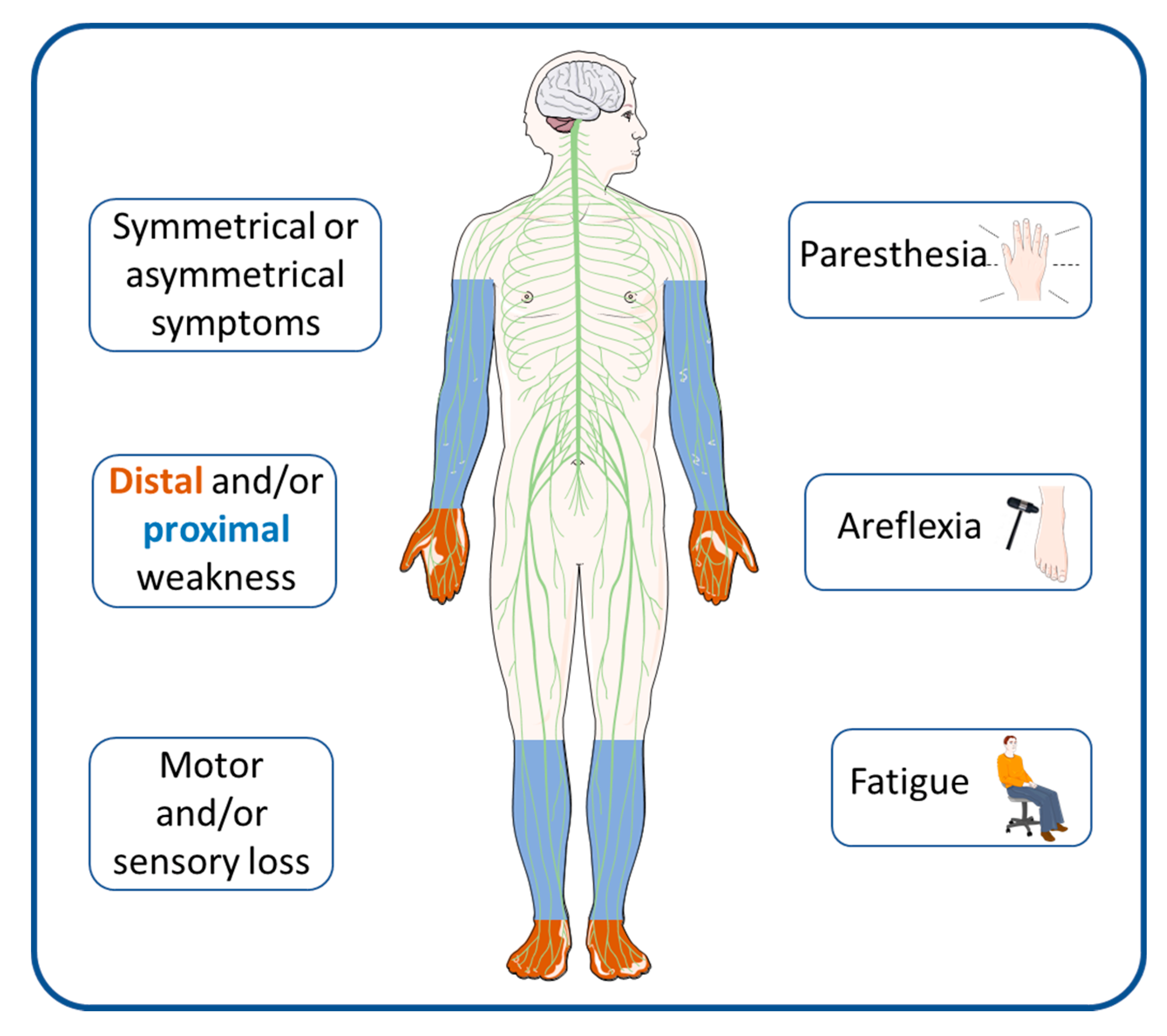
Immuno Free Full Text Cidp Current Treatments And Identification Of Targets For Future Specific Therapeutic Intervention Html

Second Intravenous Immunoglobulin Dose In Patients With Guillain Barre Syndrome With Poor Prognosis Sid Gbs A Double Blind Randomised Placebo Controlled Trial The Lancet Neurology

Pdf Restabilization Treatment After Ivig Withdrawal In Chronic Inflammatory Demyelinating Polyneuropathy Results From The Pre Randomization Phase Of The Polyneuropathy And Treatment With Hizentra Path Study Journal Of The Peripheral Nervous System

Chronic Inflammatory Demyelinating Polyneuropathy Considerations For Diagnosis Management And Population Health

The Best And Worst Ivig Side Effects You May Experience Cvidiva

Starting And Most Recent Dosing Schedule For Intravenous Immunoglobulin Download Table
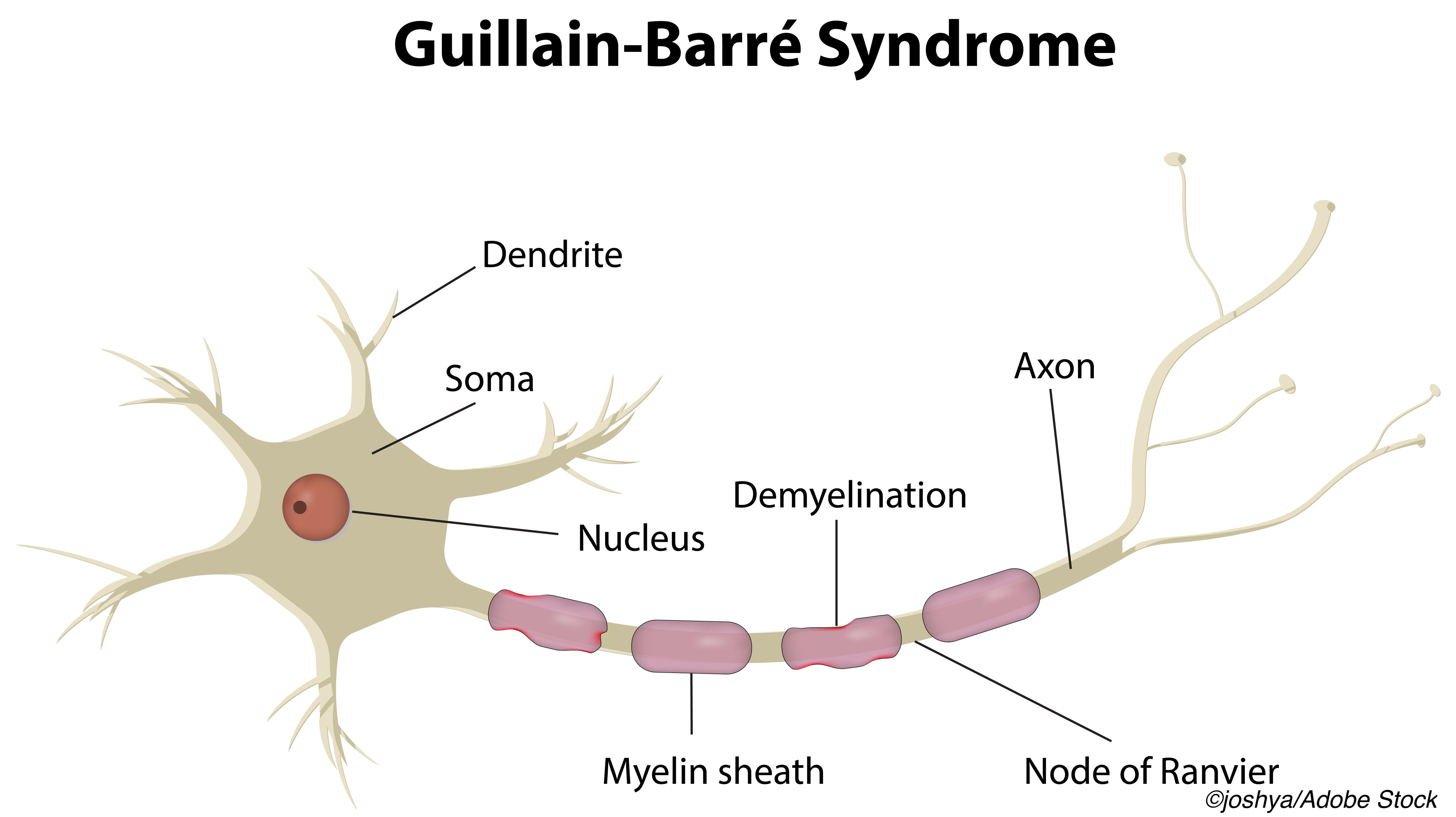
Second Ivig Course Not Helpful In Poor Prognosis Guillain Barre Syndrome Physician S Weekly

Ig Living Blog Ivig Side Effects When To Seek Medical Attention

In Cidp Is It Safe To Withdraw Ivig Advances In Chronic Inflammatory Demyelinating Polyneuropathy

Intravenous Immunoglobulin With Prednisone And Risk Adapted Chemotherapy For Children With Opsoclonus Myoclonus Ataxia Syndrome Associated With Neuroblastoma Anbl00p3 A Randomised Open Label Phase 3 Trial The Lancet Child Adolescent Health
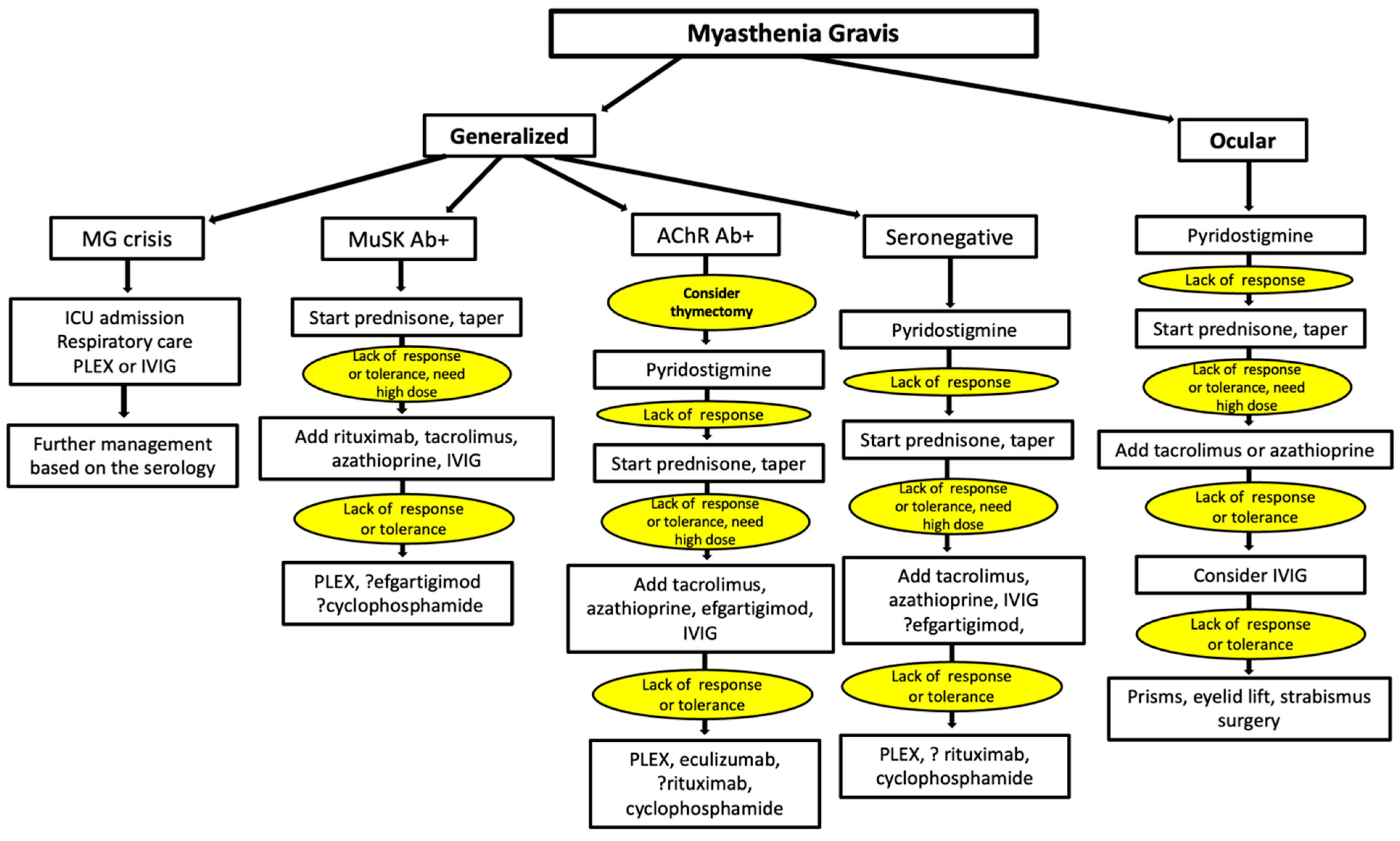
Jcm Free Full Text Current Treatment Of Myasthenia Gravis Html
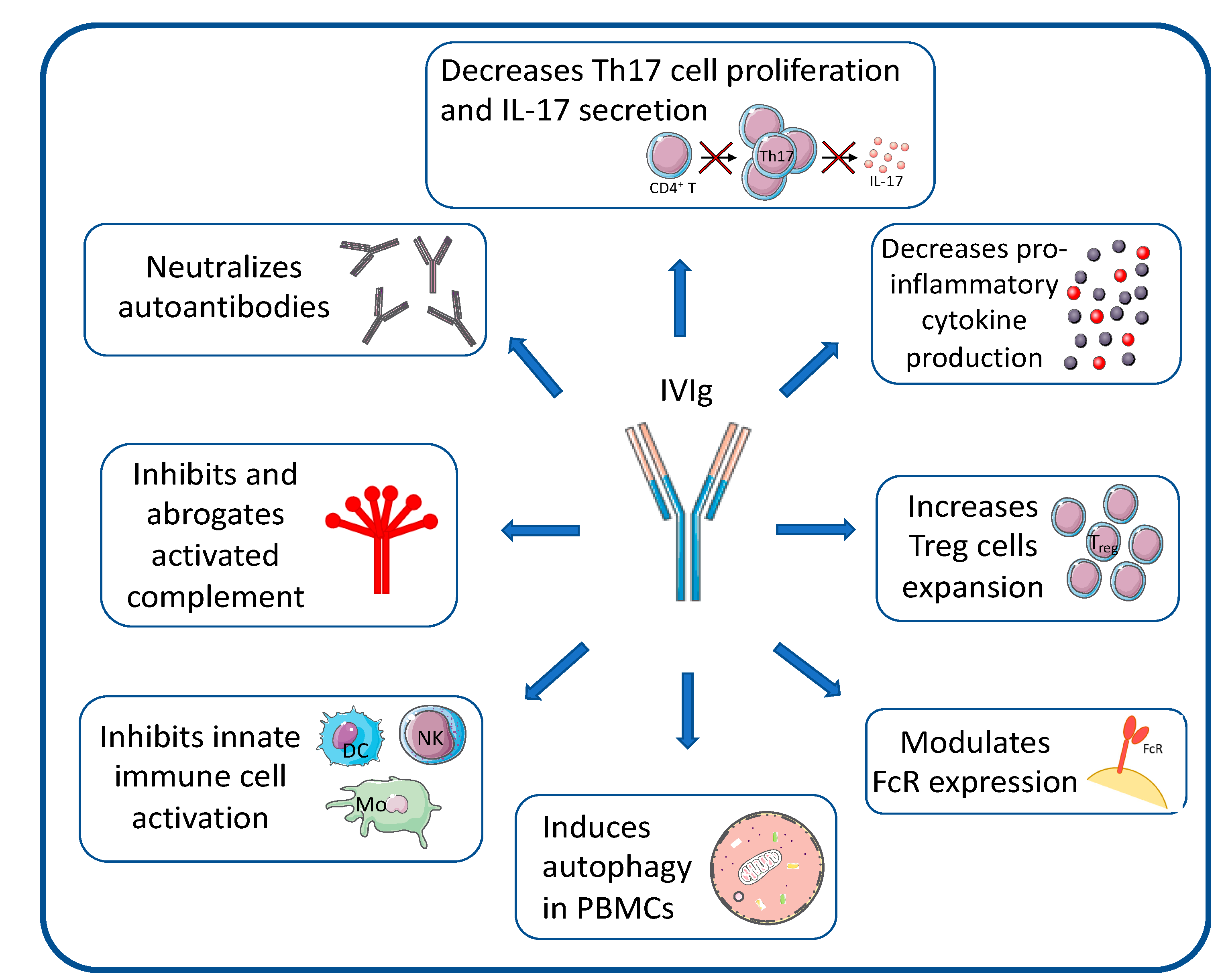
Immuno Free Full Text Cidp Current Treatments And Identification Of Targets For Future Specific Therapeutic Intervention Html

Steroid Withdrawal Syndrome Myositis Support And Understanding

Schematic Representation Of Proposed Mechanisms Of Action Of Ivig In Download Scientific Diagram